45+ calculate the formal charge of the central n
FC Formal Charge on Atom. Web Formal Charge Formula.

How To Calculate Formal Charge Chemistryscore
Calculate the formal charge of the.
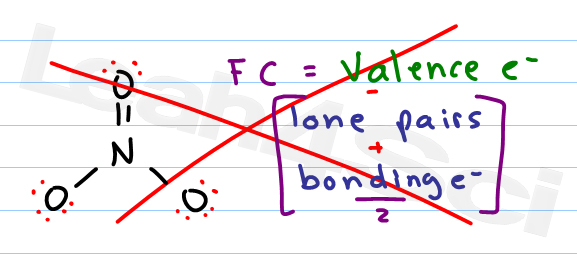
. The formal charge of an atom valence electrons of an atom non-bonding electrons ½ bonding electrons The valence electrons VE of an atom are the total number of electrons present in its valence shell. You can calculate the formal charge of any atom with the help of the equation below. L Total number of non bonding lone pair electrons in the atom.
Image transcriptions Grivey compound is. Web please give your feedback. FC V LP 5 BE Now that we know the formula let us look at the example of how to find out formal charges for individual atoms in a polyatomic molecule.
LP Lone Pair Electrons. Web In order to calculate the formal charges for CN- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec. Formal charge on central Nation 5 valence _ lone election pair 1- 4 bones electring 5 - 0 - 4 2 5-4 2.
Web The formal charges can be calculated using the formula given below. Draw the Lewis Dot Structure for the compound given in order to calculate the formal charge. Web The formal charge on the central carbon atom in the following structure is.
The formal charge of an atom valence electrons of an atom non-bonding electrons ½ bonding electrons The valence electrons VE of an atom are the total number of electrons present in its valence shell. Web For hydrocyanic acid H-C N we get the formal charges of. V Number of Valence Electrons.
Using the structure of SCN S C N ion calculate the formal charge of the sulfur atom. Web One can calculate the formal charges for any given atom with the help of the following formula. FC Valence electrons Nonbonding electrons- Bonding electrons2.
Formal charge of - No of valence election - Total no. H1-0-12 20 C4-0-12 80 N5-2-12 60 For hydrogen isocyanide H-N C we get the formal charges of H1-0-12 20 N5-0-12 81 C4-2-12 6-1 So from the above example HCN is the preferred structure isomer to HNC. Hence the formal charge on the central O atom in O3 62 1 26 1 Suggest Corrections 38 Similar questions.
Web Formal charge F C V L B 2 Where V Total number of valence electrons in the atom. N N O. Web The formal charges can be calculated using the formula given below.
Of - To fay each atom electron of love pairs around the atoy Formal charge on central Natom on NZO. B Total number of bonding shared electrons in that particular atom. Web Steps for How to Calculate Formal Charge Step 1.
FC V left LP 05BEright.

Lanthanide Single Molecule Magnets Chemical Reviews
Ochem
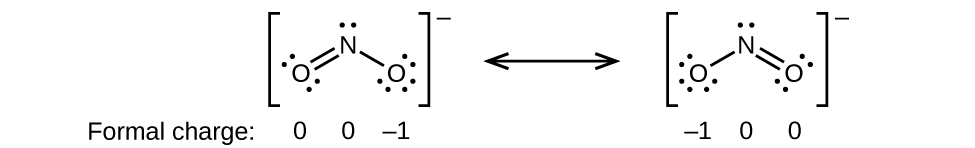
7 4 Formal Charges And Resonance Chemistry
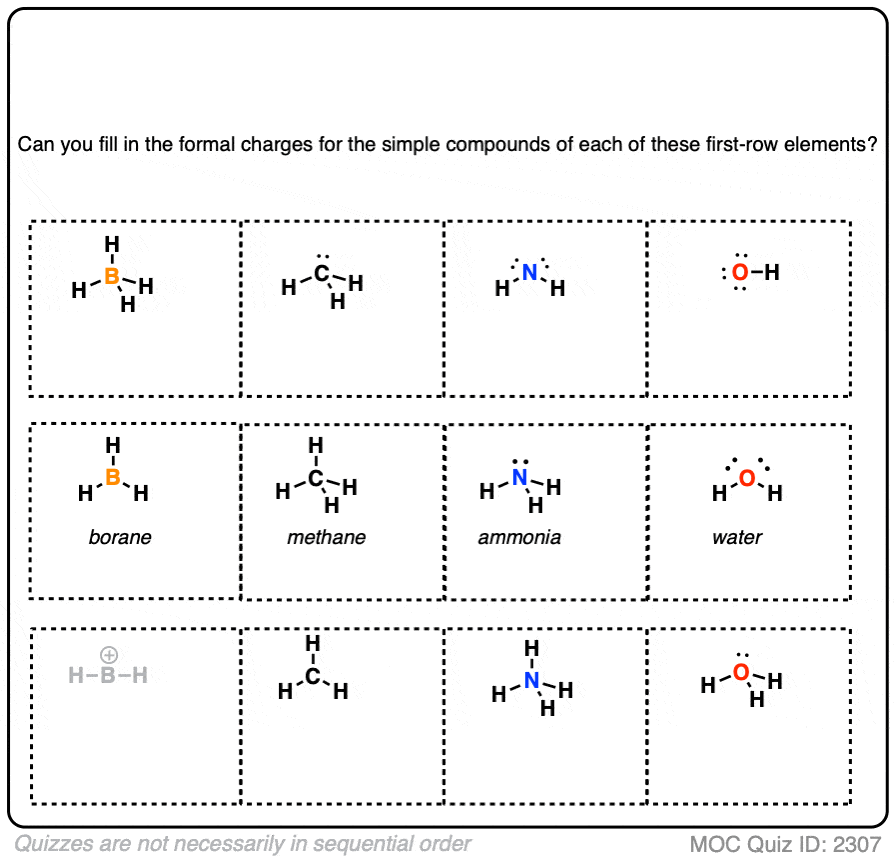
How To Calculate Formal Charge

How To Calculate Formal Charge Of An Atom In A Molecule Organic Chemistry Basics Youtube
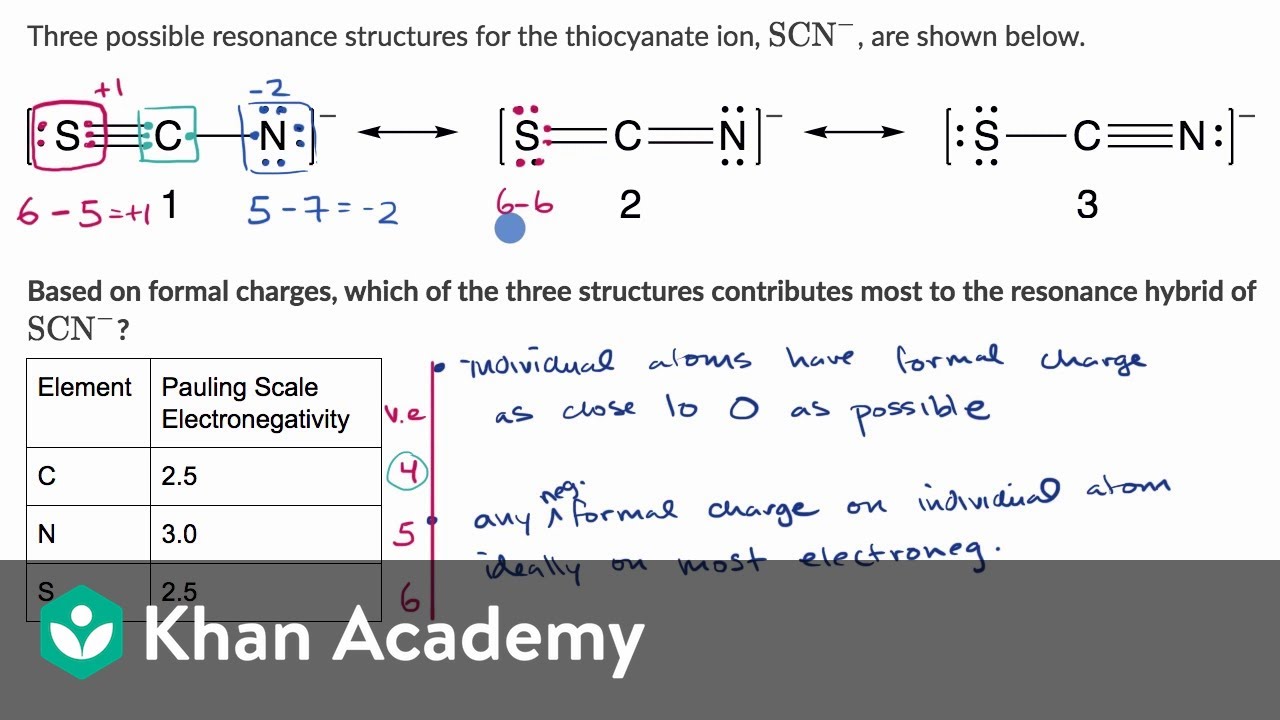
Worked Example Using Formal Charges To Evaluate Nonequivalent Resonance Structures Khan Academy Voicetube Learn English Through Videos
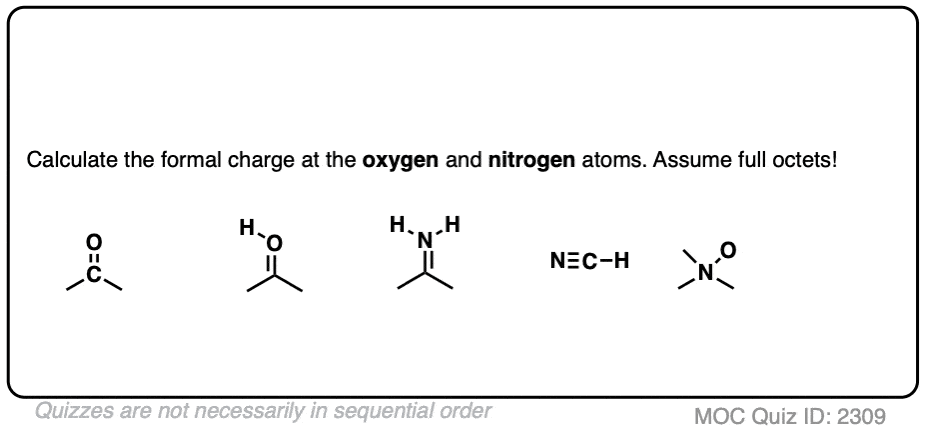
How To Calculate Formal Charge

Formal Charge Formula Calculation Shortcut For Organic Chemistry Students
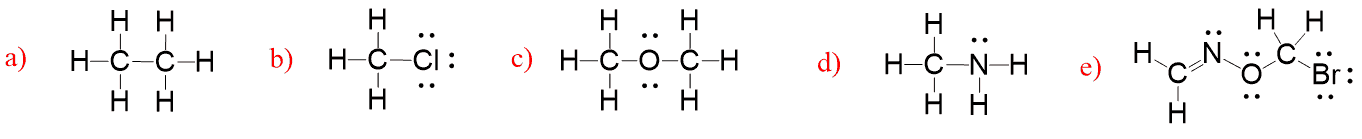
Formal Charges In Organic Chemistry Chemistry Steps

Formal Charge Formula How To Calculate Formal Charge Video Lesson Transcript Study Com

Formal Charges Calculating Formal Charge Youtube
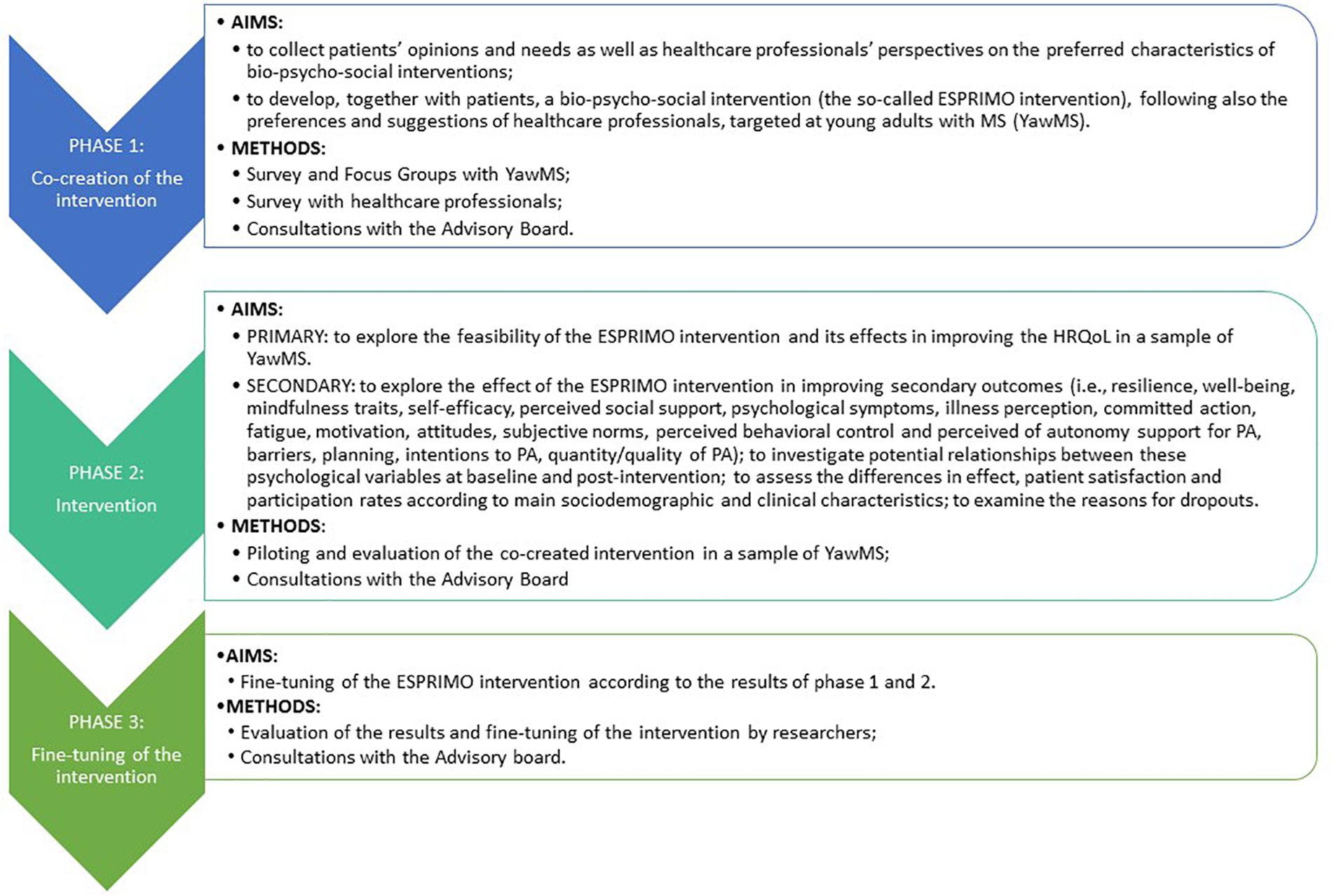
Frontiers A Bio Psycho Social Co Created Intervention For Young Adults With Multiple Sclerosis Esprimo Rationale And Study Protocol For A Feasibility Study

Formal Charges Calculating Formal Charge Youtube

Formal Charges In Organic Chemistry Chemistry Steps

Lanthanide Single Molecule Magnets Chemical Reviews
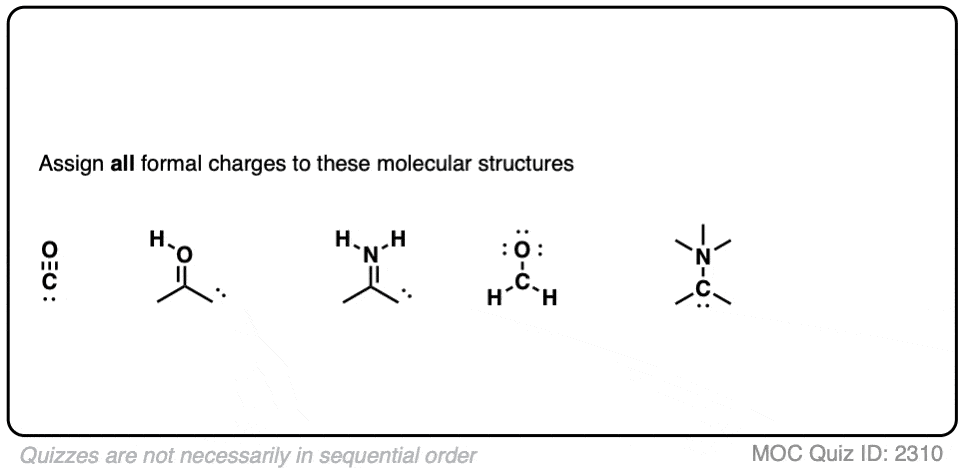
How To Calculate Formal Charge

Formal Charges In Organic Chemistry Chemistry Steps